Our range of multi-chamber bags (MCBs) has been specifically designed to meet the nutritional needs of the majority of hospitalised adult patients requiring parenteral nutrition (PN).
We have suggested MCBs from our range for clinical settings, considering national and international guidelines for nutritional intakes in specific adult patient populations. PN should be monitored, and doses adjusted according to individual patient response. Please refer to the Summary of Product Characteristics before prescribing.
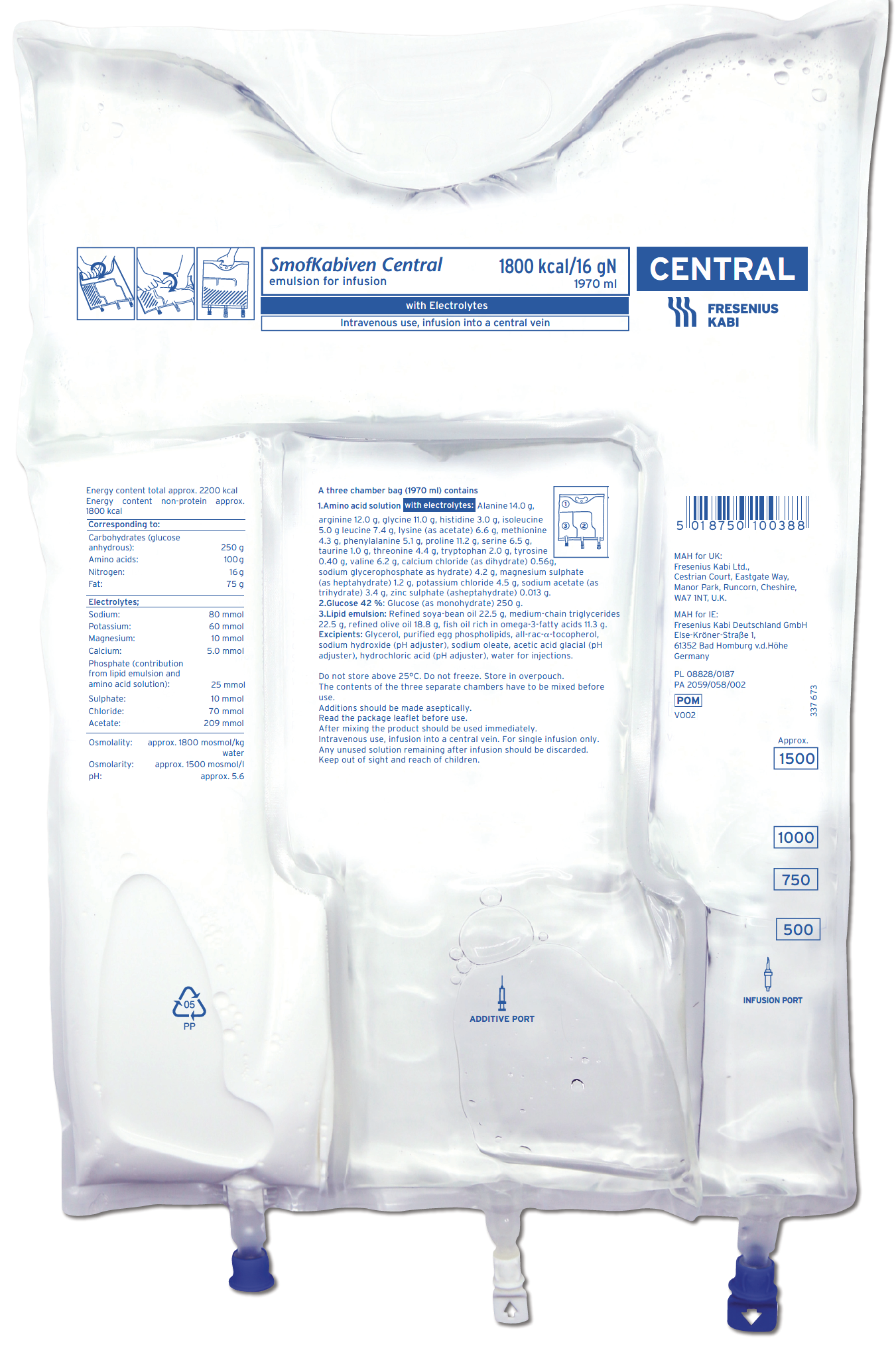
SmofKabiven Central, SmofKabiven Electrolyte Free Central and SmofKabiven Peripheral offer a well-balanced combination of nutrients that can help meet the nutritional needs of hospitalised adult patients, including those with fluid restriction.1–3 These products contain SMOFlipid®4 (soya-bean oil, medium-chain triglycerides, olive oil and fish oil), which has been shown to have less effect on liver function and to reduce hospital length of stay compared with older lipid emulsions in PN regimens.5–7
For total PN, vitamins and trace elements should be administered to the patient in addition to the macronutrients delivered via multi-chamber bags.
Figures are approximations calculated from the summaries of product characteristics.

SmofKabiven Central1,2
SmofKabiven Peripheral3
Contains SMOFlipid a four lipid emulsion
Comprises 30% soya-bean oil providing omega-6 fatty acids, 30% medium-chain triglycerides providing rapidly available energy, 25% olive oil providing omega-9 fatty acids and 15% fish oil providing omega-3 fatty acids4
Omega-3 fatty acids have proven benefits for critically ill patients.8,9 * Compared with standard ** PN, hospitalised adults who received omega 3 fatty-acid enriched PN had a:9
- 40% lower risk of infection (131 vs 215 events; RR 0.60, 95% CI 0.49–0.72; p<0.00001) – primary endpoint
- 30-day mortality rate (83 vs 101 events; RR 0.84, 95% CI 0.65–1.07; p=NS)
- 56% lower risk of sepsis (24 vs 54 events; RR 0.44, 95% CI 0.28–0.70; p=0.0004)
- Shorter ICU stay (mean stay length reduced by 1.95 days, 95% CI 0.42–3.49; p=0.01)
- Shorter hospital stay (mean stay length reduced by 2.14 days, 95% CI 1.36–2.93; p<0.00001)
Appropriate volume
SmofKabiven Central provides calories, amino acids and a four oil lipid emulsion with purified fish oil, all within an appropriate volume
- Delivers finely balanced nutrition which can help achieve nutritional targets recommended in international guidelines (with or without electrolytes), including lower volume products for fluid restricted patients10–14
Balanced protein–energy ratio
SmofKabiven Central
Protein – energy ratio: 1.2 gAA/25 kcal
Nitrogen (g) – non-protein kcal ratio: 1:108
SmofKabiven Peripheral
Protein – energy ratio: 1.3gAA/28kcal
Nitrogen (g) – non-protein kcal ratio: 1:110
- Offers a nitrogen: non-protein–energy ratio aimed at meeting the nutritional requirements of the majority of adult patients requiring PN12–17
- Provides a ratio of calories and proteins to help to avoid metabolic complications in patients receiving PN13–16
Concentrated amino acids
SmofKabiven Central provides a concentrated amount (total amino acids 51 g/1000ml) of essential, non-essential and conditionally essential amino acids1,2
Contains taurine which may have a hepatoprotective effect1,2,18
SmofKabiven Central is available with or without electrolytes1,2
- SmofKabiven Central
- SmofKabiven Electrolyte Free Central
SmofKabiven Central is available in 4 sizes.1
SmofKabiven Electrolyte Free Central is available in 3 sizes.2
SmofKabiven Peripheral is available in one size.3
SmofKabiven Central
- SmofKabiven 4 gN, 493 ml
- SmofKabiven 8 gN, 986 ml
- SmofKabiven 12 gN, 1477 ml
- SmofKabiven 16 gN, 1970 ml
SmofKabiven EF Central
- SmofKabiven EF 8 gN, 986 ml
- SmofKabiven EF 12 gN, 1477 ml
- SmofKabiven EF 16 gN, 1970 ml
SmofKabiven Peripheral
- SmofKabiven Peripheral 9.8 gN, 1904 ml
CI = confidence interval; EF = electrolyte free; gAA = grams amino acids; ICU = intensive care unit; N=nitrogen; PN = parenteral nutrition; RR = relative risk.
* This was a systematic review and meta-analysis of 49 randomised, controlled trials (3,641 patients). For the primary endpoint of infection rate, 24 studies were included (2,154 patients), 7 of these were in ICU patients and 17 in non-ICU patients. Thirty-day mortality was a co-primary outcome reported in 20 of the trials.9
** non-ω-3 fatty acid enriched.

Compared with other commercially available MCBs licensed for peripheral administration, SmofKabiven Low Osmo Peripheral, based on the kcal content per ml of fluid, and maximum hourly infusion rates and recommended infusion periods for adults, can deliver the most kcal per kg bw/hour.1* It contains SMOFlipid® (soya-bean oil, medium-chain triglycerides, olive oil and fish oil),2 which may have positive effects on patient outcomes.3–6
SmofKabiven Low Osmo Peripheral has the lowest osmolarity, compared with other commercially available MCBs licensed for peripheral administration,1,7–10 within expert guideline recommendations for osmolarities for peripheral PN.11,12*
For total PN, vitamins and trace elements should be administered to the patient in addition to the macronutrients delivered via multi-chamber bags.
Figures are approximations calculated from the summaries of products characteristics.

SmofKabiven Low Osmo Peripheral1
Contains SMOFlipid, a four lipid emulsion2
SmofKabiven Low Osmo Peripheral contains the same SMOFlipid found in all the SmofKabiven ranges
- Shown to have less impact on liver cell function and inflammatory response versus older lipid emulsions3,5,13–15
Contains Aminoven® 16 (amino acids 10%)
SmofKabiven Low Osmo Peripheral provides essential, non-essential and conditionally essential amino acids
- Contains taurine which may have a hepatoprotective effect17
Efficient energy delivery
SmofKabiven Low Osmo Peripheral provides the most kcal/kg/hour compared with other commercially available MCBs licensed for peripheral use when maximum infusion rates and recommended infusion periods for adults are applied.1,7-10
- Lowest osmolarity compared with other commercially available MCBs licensed for peripheral administration*1,7-10
For short-term PN
SmofKabiven Low Osmo Peripheral1 is designed to meet the needs of patients requiring short-term nutrition support and for whom energy needs are prioritised
- Peripheral venous access can be quicker to establish than central venous access, offering the possibility for prompt administration of PN18,19
- Helps support venous tolerability through low osmolarity11,12,18–21
SmofKabiven Low Osmo Peripheral available in 3 sizes1
- SmofKabiven Low Osmo Peripheral 6 gN, 1400 ml
- SmofKabiven Low Osmo Peripheral 8 gN, 1950 ml
- SmofKabiven Low Osmo Peripheral 10 gN, 2500 ml
ASPEN = American Society for Parenteral and Enteral Nutrition; ESPEN = European Society for Clinical Nutrition and Metabolism; gAA = grams amino acids; MCB: multi-chamber bag; N = nitrogen; PN = parenteral nutrition
*PN osmolarity: <850 mosmol/l according to ESPEN guidelines;11 <900 mosmol/l according to ASPEN guidelines;12
Compared with commercially available products: Triomel Peripheral (Baxter Healthcare)7, Lipoflex Peri (B Braun)8, Finomel Peri (Baxter Healthcare)9, Omeflex Peri (B Braun)10

SMOFlipid 200 mg/ml is an established four oil lipid mix containing 30% soya-bean oil, 30% medium-chain triglycerides (MCT), 25% olive oil and 15% fish oil.1 It provides energy and essential fatty acids for parenterally fed patients.
Omega-3 fatty acids in parenteral nutrition (PN) have proven benefits for critically ill patients.2 SMOFlipid was the most purchased lipid emulsion brand for parenteral nutrition in the UK in 2020, according to Data Alliance – UK intravenous lipid emulsion sales by unit 2020.3
Find out more about SMOFlipid here: https://multichamberbags.co.uk/smoflipid
SMOFlipid1
Four lipid emulsion
SMOFlipid comprises 30% soya-bean oil providing omega-6 fatty acids, 30% medium-chain triglycerides providing rapidly available energy, 25% olive oil providing omega-9 fatty acids and 15% fish oil providing omega-3 fatty acids1
- Appropriate amount of lipids
the dose for adults of 1.0–2.0g fat/kg body weight/day (5–10ml/kg body weight/day);1 provides 0.15–0.3g fish oil/kg body weight/day; an omega-6/omega-3 fatty acid ratio of approximately 2.5:14 - As an individual PN component, can be used to meet recommendations of a maximum of 1.5g lipids/kg/day in critically ill adult patients5 where PN is indicated
Less disruption to markers of immune and metabolic function
compared with standard lipid emulsions even with short-term use2,4,6,7
- Lower levels of proinflammatory markers compared with an olive/soya-bean oil emulsion and a soya-bean oil emulsion in adult ICU patients4,8
- Reduces omega-6 fatty acid load compared to lipid emulsions based on soya-bean oil2,9
Less disruption to markers of liver function compared with standard lipid emulsion after four weeks10
- Less effects on parameters of liver function compared with an olive/soya-bean oil emulsion and a soya-bean emulsion6,7
Omega-3 fatty acids have proven benefits for critically ill patients2,11*
* Compared with standard† PN, hospitalised adults who received omega 3 fatty-acid enriched PN had a:2
- 40% lower risk of infection (131 vs 215 events; RR 0.60, 95% CI 0.49–0.72; p<0.00001) – Primary endpoint
- 30-day mortality rate (83 vs 101 events; RR 0.84, 95% CI 0.65–1.07; p=NS)
- 56% lower risk of sepsis (24 vs 54 events; RR 0.44, 95% CI 0.28–0.70; p=0.0004)
- Shorter ICU stay (mean stay length reduced by 1.95 days, 95% CI 0.42–3.49; p=0.01)
- Shorter hospital stay (mean stay length reduced by 2.14 days, 95% CI 1.36–2.93; p<0.00001)
CI = confidence interval; ICU = intensive care unit; gAA = grams amino acids; NS = not significant; PN = parenteral nutrition.
* This was a systematic review and meta-analysis of 49 randomised, controlled trials (3,641 patients). For the primary endpoint of infection rate, 24 studies were included (2,154 patients), 7 of these were in ICU patients and 17 in non-ICU patients. Thirty-day mortality was a co-primary outcome reported in 20 of the trials.2
†non-ω-3 fatty acid enriched


